
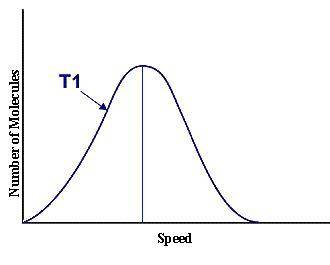
This graph represents a population of molecules in a gas versus the distribution of the average velocity(speed) of its molecules in that population. Assume all molecules to be of the same mass. In reading the graph, it is important to note three things. One, is the most probable speed is at the peak of the curve. Secondly, the most probable speed increases as the temperature increases (so shift to the right), and the distribution broadens as it increases.
On the graph, indicate the average kinetic energy of the population.
Explain your answer.
What part of the graph indicates the temperature of the sample?
Explain your answer.
Print out graph paper (click here for graph paper) and sketch a curve that represents the distribution of molecules at a temperature below the one shown. Label it as T2. Describe both T and T2 in terms of their average kinetic energy. Be specific and detailed.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 23.06.2019 07:30, sweetLips230
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1

Chemistry, 23.06.2019 13:30, arianasg06
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3

Chemistry, 23.06.2019 22:40, bicaplinger7766
For the following reaction, 3.70 grams of oxygen gas are mixed with excess carbon monoxide . the reaction yields 8.25 grams of carbon dioxide . carbon monoxide(g) + oxygen(g) carbon dioxide(g) what is the ideal yield of carbon dioxide? grams what is the percent yield for this reaction? %
Answers: 1
Do you know the correct answer?
This graph represents a population of molecules in a gas versus the distribution of the average velo...
Questions in other subjects:


Chemistry, 05.05.2020 07:41


Health, 05.05.2020 07:41

Mathematics, 05.05.2020 07:41



Mathematics, 05.05.2020 07:41

English, 05.05.2020 07:41

Social Studies, 05.05.2020 07:41






