
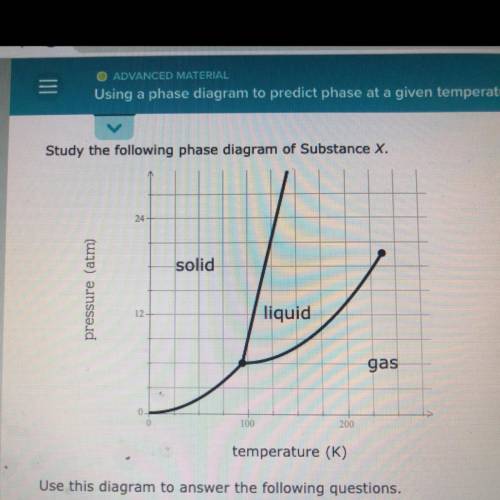
Use this diagram to answer the following questions.
Suppose a small sample of pure X is held at -48. °C and 10.7 atm.
What will be the state of the sample?
(choose one)
Suppose the temperature is held constant at -48. °C but the pressure
is increased by 3.2 atm. What will happen to the sample?
(choose one)
Suppose, on the other hand, the pressure is held constant at 10.7 atm
but the temperature is decreased by 80. °C. What will happen to the
sample?
(choose
one)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 06:00, VamPL
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl +4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 23.06.2019 00:50, kaseywright3418
Which statement would indicate the presence of an acid
Answers: 3
Do you know the correct answer?
Use this diagram to answer the following questions.
Suppose a small sample of pure X is held at -4...
Questions in other subjects:

Mathematics, 06.12.2021 03:50

Mathematics, 06.12.2021 04:00

English, 06.12.2021 04:00

English, 06.12.2021 04:00

Advanced Placement (AP), 06.12.2021 04:00

Mathematics, 06.12.2021 04:00


Mathematics, 06.12.2021 04:00







