Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1


Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1
Do you know the correct answer?
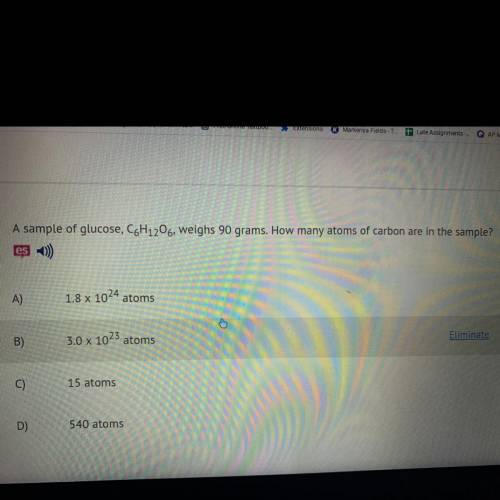
A sample of glucose, C6H12O6, weighs 90 grams. How many atoms of carbon are in the sample?
A)
Questions in other subjects:


Chemistry, 19.04.2021 20:10


Mathematics, 19.04.2021 20:10




Arts, 19.04.2021 20:10