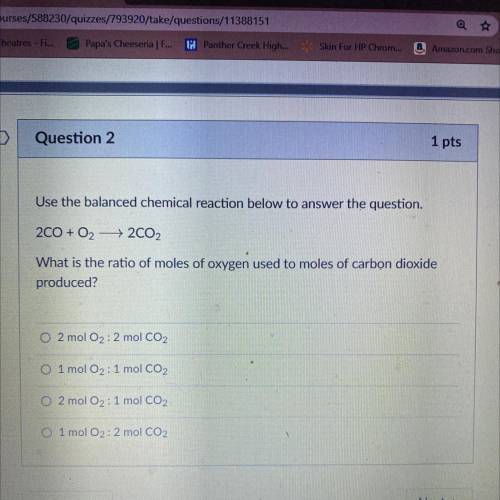
Question 2
Use the balanced chemical reaction below to answer the question.
200 + O2 + 2CO2<...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
Do you know the correct answer?
Questions in other subjects:

English, 17.04.2022 01:40




Mathematics, 17.04.2022 01:50