
Chemistry, 26.04.2021 07:40, kinglightskin2k
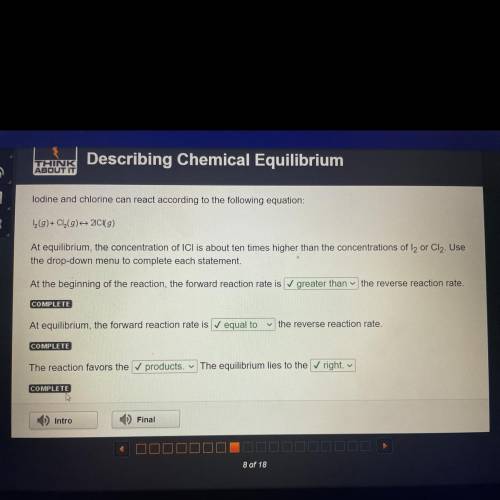
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At equilibrium, the concentration of ICI is about ten times higher than the concentrations of l2 or Cl2. Use
the drop-down menu to complete each statement.
At the beginning of the reaction, the forward reaction rate is greater than the reverse reaction rate.
COMPLETE
At equilibrium, the forward reaction rate is equal to
the reverse reaction rate.
COMPLETE
The reaction favors the ✓ products. The equilibrium lies to the right.
COMPLETE

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1


Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
Do you know the correct answer?
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At eq...
At eq...
Questions in other subjects:

Mathematics, 16.02.2021 16:10



Biology, 16.02.2021 16:10

Mathematics, 16.02.2021 16:10

English, 16.02.2021 16:10

Mathematics, 16.02.2021 16:10

Mathematics, 16.02.2021 16:10

English, 16.02.2021 16:10






