Chemistry, 26.04.2021 02:20, dezmondpowell
PLZZZ HELP
The strong intermolecular forces in water lead to strong surface tension, as each water molecule exerts a high force on neighboring molecules. Because the molecules on the surface have fewer neighboring molecules, they are held more tightly to the molecules beneath them. These strong forces will allow water to resist evaporation on the surface of the body of water, to form nearly spherical raindrops, and to move upward through plants.
How would our planet be different if water only had London dispersion forces holding the molecules together? Give three specific examples

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, natannale
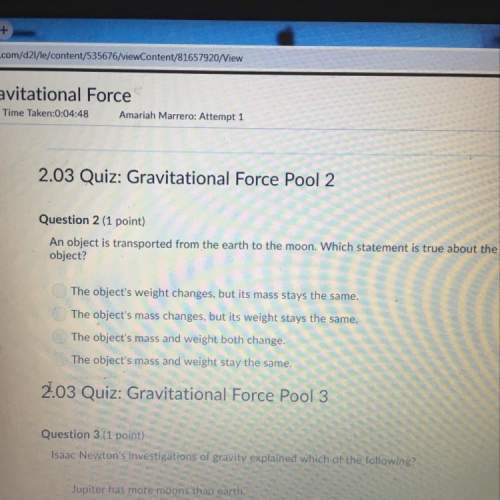
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1


Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
Do you know the correct answer?
PLZZZ HELP
The strong intermolecular forces in water lead to strong surface tension, as each water...
Questions in other subjects:


Mathematics, 03.10.2019 03:30

Chemistry, 03.10.2019 03:30


English, 03.10.2019 03:30

English, 03.10.2019 03:30



Mathematics, 03.10.2019 03:30

Mathematics, 03.10.2019 03:30