
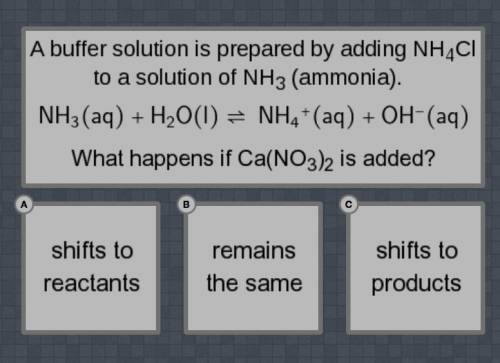
A buffer solution is prepared by adding NH4Cl to a solution of NH3 (ammonia).
NH3(aq) + H2O(I) = NH4+(aq) +OH-(aq0
What happens if Ca(NO3)2 is added?
Shifts to reactants, remain the same, shifts to products.
Please HELP!
I watched the video, but I still don't get it!
I don't know how this works...

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1


Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
Do you know the correct answer?
A buffer solution is prepared by adding NH4Cl to a solution of NH3 (ammonia).
NH3(aq) + H2O(I) = N...
Questions in other subjects:




Chemistry, 25.09.2021 08:00

Social Studies, 25.09.2021 08:00


Mathematics, 25.09.2021 08:00


English, 25.09.2021 08:00

Advanced Placement (AP), 25.09.2021 08:00






