PLEASEEE HELP A chemist mixed two substances
together: a blue powder with no smell
and a col...

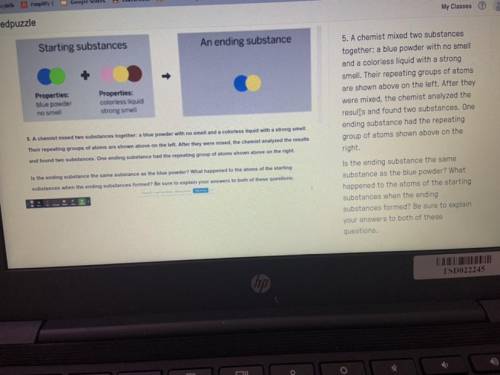
PLEASEEE HELP A chemist mixed two substances
together: a blue powder with no smell
and a colorless liquid with a strong
smell. Their repeating groups of atoms
are shown above on the left. After they
were mixed, the chemist analyzed the
results and found two substances. One
ending substance had the repeating
group of atoms shown above on the
right.
Is the ending substance the same
substance as the blue powder? What
happened to the atoms of the starting
substances when the ending
substances formed? Be sure to explain
your answers to both of these

Answers: 2
Other questions on the subject: Chemistry



Do you know the correct answer?
Questions in other subjects:







Mathematics, 20.08.2019 21:30

History, 20.08.2019 21:30

Mathematics, 20.08.2019 21:30

History, 20.08.2019 21:30






