
Chemistry, 23.04.2021 22:40, ibarral37102
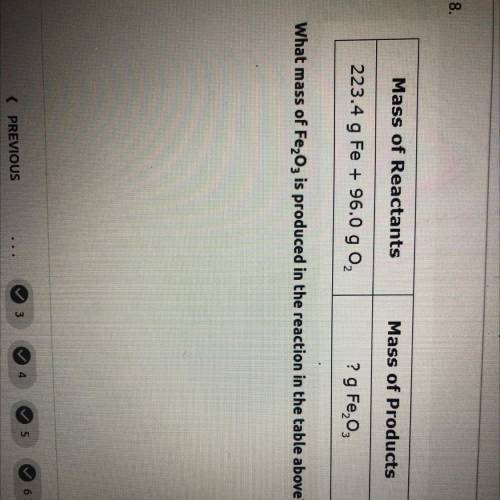
what mass of Fe2O3 is produced in the reaction in the table above mass of reactants= 223.4 g Fe+96.0 g O2. and mass of products=? g Fe2O3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2


Chemistry, 23.06.2019 14:20, baler19
Timed ! in which of these statements are protons, electrons, and neutrons correctly compared? quarks are present in protons and neutrons but not in electrons. quarks are present in protons, neutrons, and electrons. quarks are present in neutrons and electrons but not in protons. quarks are present in protons and electrons but not in neutrons.
Answers: 1
Do you know the correct answer?
what mass of Fe2O3 is produced in the reaction in the table above mass of reactants= 223.4 g Fe+96.0...
Questions in other subjects:


Biology, 23.01.2020 20:31



Mathematics, 23.01.2020 20:31



Health, 23.01.2020 20:31







