
Chemistry, 23.04.2021 04:20, tashanicole
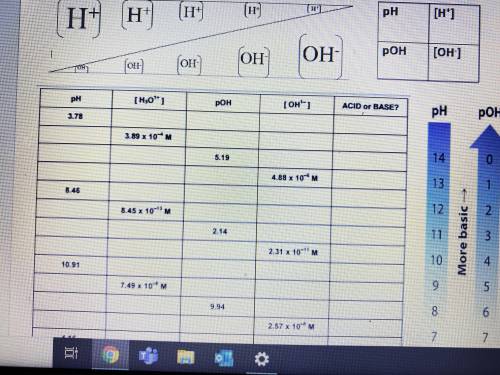
So I have half of the chart filled out, I just need the answers for each column and row starting from pH 8.46 (5th one in the first column) all the way to OH 2.47*10^-8 hope it kinda makes since
please help
NO LINKS PLEASE

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 06:00, hopechinn6646
Complete the sentences to best explain the ranking. match the words below to the appropriate blanks in the sentences. a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1

Chemistry, 23.06.2019 08:00, kyle65
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2

Chemistry, 23.06.2019 14:00, Gaby702
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 6.00 mol fe and 8.45 mol nio(oh) react?
Answers: 1
Do you know the correct answer?
So I have half of the chart filled out, I just need the answers for each column and row starting fro...
Questions in other subjects:


English, 05.05.2020 11:52

Mathematics, 05.05.2020 11:52




Mathematics, 05.05.2020 11:52








