2 H202 - 2 H2O + O2

Chemistry, 22.04.2021 04:40, cjasmine626
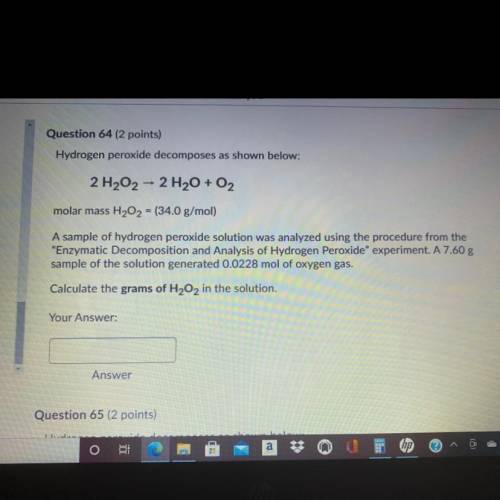
Question 64 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
molar mass H202 = (34.0 g/mol)
A sample of hydrogen peroxide solution was analyzed using the procedure from the
"Enzymatic Decomposition and Analysis of Hydrogen peroxide" experiment. A 7.60 g
sample of the solution generated 0.0228 mol of oxygen gas.
Calculate the grams of H2O2 in the solution.
Your
Answer

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1

Chemistry, 23.06.2019 07:00, mahogany1956
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
Do you know the correct answer?
Question 64 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
2 H202 - 2 H2O + O2
Questions in other subjects:

History, 12.11.2020 19:10


English, 12.11.2020 19:10


History, 12.11.2020 19:10



Mathematics, 12.11.2020 19:10


Mathematics, 12.11.2020 19:10






