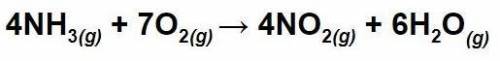Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 21.06.2019 19:30, viktoria1198zz
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2
Do you know the correct answer?
How many grams of ammonia will be required to produce 12.0g of water given the balanced chemical equ...
Questions in other subjects:


Mathematics, 30.04.2021 18:50

Mathematics, 30.04.2021 18:50

Mathematics, 30.04.2021 18:50

Mathematics, 30.04.2021 18:50

Mathematics, 30.04.2021 18:50

Mathematics, 30.04.2021 18:50

Mathematics, 30.04.2021 18:50


Mathematics, 30.04.2021 18:50