Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
Do you know the correct answer?
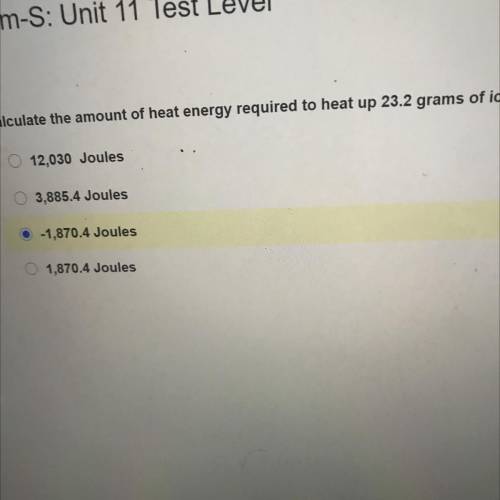
Chem-S: Unit 11 Test Level
Calculate the amount of heat energy required to heat up 23.2 grams of i...
Questions in other subjects:



Mathematics, 13.12.2021 04:20


Social Studies, 13.12.2021 04:20


Mathematics, 13.12.2021 04:20


Mathematics, 13.12.2021 04:20

Mathematics, 13.12.2021 04:20