Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
Do you know the correct answer?
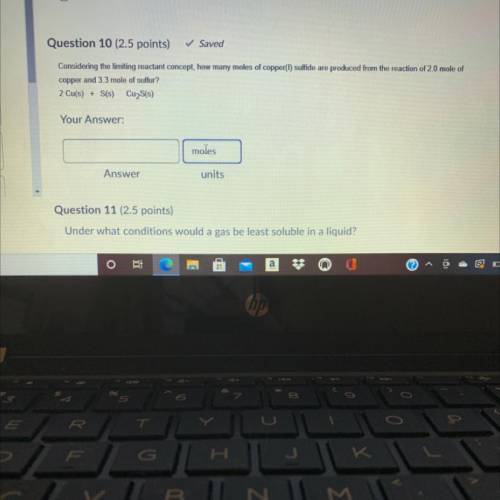
Considering the limiting reactant concept, how many moles of copper(t) sulfide are produced from the...
Questions in other subjects:

English, 04.04.2020 18:30

Geography, 04.04.2020 18:30

Mathematics, 04.04.2020 18:30



English, 04.04.2020 18:30


Mathematics, 04.04.2020 19:41


English, 04.04.2020 19:41