
Chemistry, 20.04.2021 04:20, chutchinson256
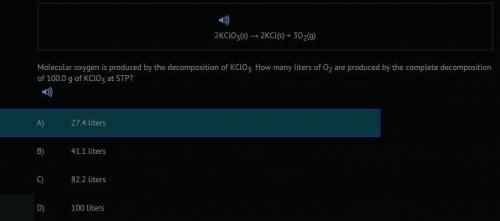
2KCLO3(s) --> 2KCL(s) + 302(g) Molecular oxygen is produced by the decomposition of KClO3. How many liters of O2 are produced by the complete decomposition of 100.0 g of KCLO3 at STP?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
Do you know the correct answer?
2KCLO3(s) --> 2KCL(s) + 302(g) Molecular oxygen is produced by the decomposition of KClO3. How ma...
Questions in other subjects:

Chemistry, 28.07.2020 20:01

Mathematics, 28.07.2020 20:01

Mathematics, 28.07.2020 20:01



Mathematics, 28.07.2020 20:01

Health, 28.07.2020 20:01


Chemistry, 28.07.2020 20:01

Mathematics, 28.07.2020 20:01






