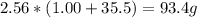2nacl + h2so4 --> 2hcl + na2so4
how many grams of hcl can be prepared from 2.00 mol h2so4...

Chemistry, 23.10.2019 03:00, julionavedo21
2nacl + h2so4 --> 2hcl + na2so4
how many grams of hcl can be prepared from 2.00 mol h2so4 and 2.56 mol nacl?
a. 7.30 g
b. 93.3 g
c. 146 g
d. 150 g
e. 196 g

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 01.07.2019 14:30

Mathematics, 01.07.2019 14:30



English, 01.07.2019 14:30




Mathematics, 01.07.2019 14:30


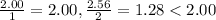 hence NaCl is the limiting reagant.
hence NaCl is the limiting reagant.