Chemistry, 16.09.2019 10:30, asseatingbandit
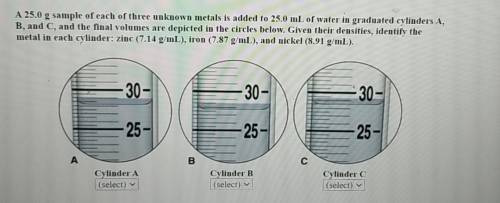
44 a 25.0-g sample of each of three unknown metals is added to 25.0 ml of water in graduated cylinders a, b, and c, and the final volumes are depicted in the circles below. given their densities, identify the metal in each cylinder: zinc (7.14 g/ml), iron (7.87 g/ml), or nickel (8.91 g/ml).

Answers: 1
Similar questions

Chemistry, 03.07.2019 01:00, jetblackcap
Answers: 1


Chemistry, 13.09.2019 08:20, kennakenken3
Answers: 1

Biology, 13.09.2019 09:10, SoWhat2237
Answers: 2
Do you know the correct answer?
44 a 25.0-g sample of each of three unknown metals is added to 25.0 ml of water in graduated cylinde...
Questions in other subjects:


Mathematics, 16.01.2021 04:40

Mathematics, 16.01.2021 04:40

History, 16.01.2021 04:40



English, 16.01.2021 04:40


Mathematics, 16.01.2021 04:40