Given the redox reaction:
cr3+ + al--> cr + al3+
as the reaction takes place, there...


Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Biology, 20.09.2020 04:01

History, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01




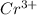
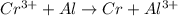
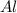 to
to 



