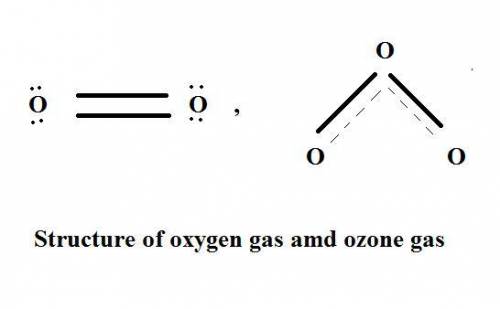
Which statement explains why ozone gas, o3, and oxygen gas, o2, have different properties?
(1...

Chemistry, 30.09.2019 10:30, sharnisefrazier
Which statement explains why ozone gas, o3, and oxygen gas, o2, have different properties?
(1) they are formed from different elements.
(2) they have different molecular structures.
(3) they have different oxidation numbers.
(4) they have different electronegativities.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Spanish, 06.12.2020 18:30

Mathematics, 06.12.2020 18:30


Chemistry, 06.12.2020 18:30

English, 06.12.2020 18:30


History, 06.12.2020 18:30

History, 06.12.2020 18:30

Mathematics, 06.12.2020 18:30

Mathematics, 06.12.2020 18:30


 (Paramagnetic)
(Paramagnetic) (diamagnetic)
(diamagnetic)