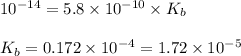Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3


Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
Do you know the correct answer?
The acid dissociation constant for boric acid, h3bo3, is 5.8 x 10-10. calculate the dissociation con...
Questions in other subjects:

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01



English, 13.10.2020 05:01

Health, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Biology, 13.10.2020 05:01

Engineering, 13.10.2020 05:01


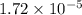
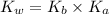
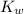 = Ionic product of water =
= Ionic product of water = 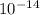
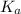 = Acid dissociation constant =
= Acid dissociation constant = 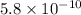
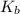 Base dissociation constant = ?
Base dissociation constant = ?