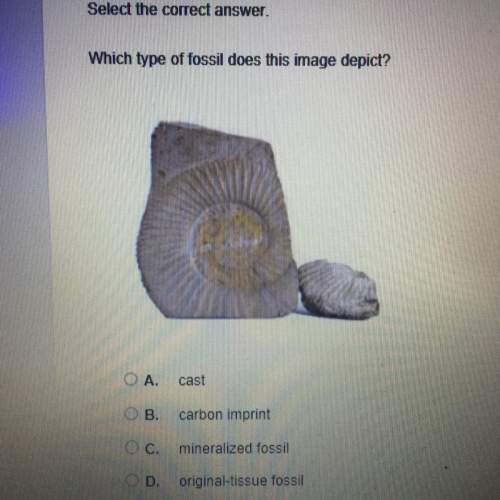
Chemistry, 24.09.2019 09:00, indiaseel5940
Copper has two stable isotopes 63cu and 65cu. 63cu has an abundance of 69.09%. the other isotope, 65cu, has an abundance of 30.91%. calculate the average atomic mass of copper.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1


Chemistry, 23.06.2019 05:40, girlchamp654
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 10:30, aliyyahlove
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1
Do you know the correct answer?
Copper has two stable isotopes 63cu and 65cu. 63cu has an abundance of 69.09%. the other isotope, 65...
Questions in other subjects:

Mathematics, 22.02.2021 18:30

Biology, 22.02.2021 18:30

Mathematics, 22.02.2021 18:30

Mathematics, 22.02.2021 18:30



English, 22.02.2021 18:30


Mathematics, 22.02.2021 18:30