
Chemistry, 01.09.2019 02:30, nulledcracker12
Consider the balanced chemical equation that follows. you are asked to determine how many moles of water you can produce from 4.0 mol of hydrogen and excess oxygen. (excess oxygen means that so much oxygen is available it will not run out.) which of the numbers that appear in the balanced chemical equation below are used to perform this calculation? 2h2(g)+o2(g)→2h2o(l)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
Do you know the correct answer?
Consider the balanced chemical equation that follows. you are asked to determine how many moles of w...
Questions in other subjects:

Mathematics, 30.10.2020 03:10




Mathematics, 30.10.2020 03:10


Business, 30.10.2020 03:10


Geography, 30.10.2020 03:10

History, 30.10.2020 03:10


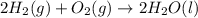
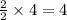 moles of water
moles of water



