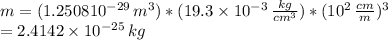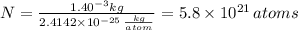Chemistry, 23.09.2019 05:30, robloxlover1987
The atomic radius of a gold atom is 144×10^-12. the volume of a gold atom can be calculated using the volume of a sphere. the density of gold is 19.3 g/cm^3. how many atoms are present in a sample of gold with a mass of 1.40g using the info provided

Answers: 1
Similar questions



Chemistry, 30.09.2019 08:30, ayalat9596
Answers: 1
Do you know the correct answer?
The atomic radius of a gold atom is 144×10^-12. the volume of a gold atom can be calculated using th...
Questions in other subjects:



History, 26.08.2019 09:30



Mathematics, 26.08.2019 09:30


English, 26.08.2019 09:30

History, 26.08.2019 09:30