
Chemistry, 29.01.2020 22:57, mauricioperez0902
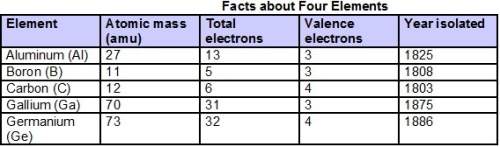
Based on the information in the table, which elements are most likely in the same periods of the periodic table?
a. boron and carbon are likely together in one period because they have very close atomic numbers, while gallium and germanium are likely together in another period because they have very close atomic numbers.
b. aluminum, boron, and carbon are likely together in one period because they were first isolated in the first half of the 1800s, while gallium and germanium are likely together in another period because they were first isolated in the second half.
c. boron and carbon are likely together in one period because they each end in “-on,” while aluminum, gallium, and germanium are likely together in another period because they each end in “-ium.”
d. aluminum, boron, and gallium are likely together in one group because they have the same number of valence electrons, and carbon and germanium are likely together in another group because they have the same number of valence electrons.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
Do you know the correct answer?
Based on the information in the table, which elements are most likely in the same periods of the per...
Questions in other subjects:




History, 14.12.2020 18:20



Mathematics, 14.12.2020 18:20

Mathematics, 14.12.2020 18:20







