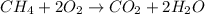Chemistry, 11.10.2019 10:50, rebecca7415
Methane (ch4) is the main component of natural gas. it is burned for fuel in a combustion reaction. the unbalanced combustion reaction for methane is shown below. ch4 + o2 co2 + h2o + heat when the reaction is balanced, how many carbon dioxide molecules are produced for every methane molecule burned?

Answers: 1
Similar questions

Chemistry, 09.08.2019 16:20, estheradame547
Answers: 2

Chemistry, 05.09.2019 19:30, ErikHabdlowich
Answers: 1

Chemistry, 01.10.2019 16:30, rexard
Answers: 1
Do you know the correct answer?
Methane (ch4) is the main component of natural gas. it is burned for fuel in a combustion reaction....
Questions in other subjects:






Mathematics, 05.05.2020 19:00