
Chemistry, 04.02.2020 05:01, lalala1212
1.) why does carbon form covalent bonds?
it tends to pull 4 electrons from another atom.
it tends to release 4 electrons to another atom.
it will usually bond to multiple atoms which can provide a total of 4 additional electrons.
it has a strong electronegativity.
2.) what type of bond will be formed for atoms that have a +1 or -1 charge?
covalent
ionic
they do not react
elemental
3.) select all that apply.
the relative ionic or covalent character of chemical bonds may be estimated by:
the total number of electrons for each element
the addition of electronegativities
comparison of the associated families to which the elements belong
the difference in electronegativities

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1


Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1
Do you know the correct answer?
1.) why does carbon form covalent bonds?
it tends to pull 4 electrons from another atom...
it tends to pull 4 electrons from another atom...
Questions in other subjects:

Mathematics, 04.11.2020 02:10




Mathematics, 04.11.2020 02:10

Mathematics, 04.11.2020 02:10

History, 04.11.2020 02:10


Mathematics, 04.11.2020 02:10

Mathematics, 04.11.2020 02:10


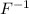 ion and sodium on losing an electron will form a
ion and sodium on losing an electron will form a 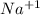 ion.
ion.



