
One way the u. s. environmental protection agency (epa) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with copper(ii) chloride, which would react with silver nitrate solution like this: cucl2 (aq) + 2agno3 (aq) → 2agcl (s) + cuno32 (aq) the chemist adds 26.0m m silver nitrate solution to the sample until silver chloride stops forming. she then washes, dries, and weighs the precipitate. she finds she has collected 6.1mg of silver chloride. calculate the concentration of copper(ii) chloride contaminant in the original groundwater sample. be sure your answer has the correct number of significant digits.

Answers: 2
Similar questions

Chemistry, 10.08.2019 00:30, kaitlynk0
Answers: 3

Chemistry, 01.10.2019 02:10, zandariouslee
Answers: 2
Do you know the correct answer?
One way the u. s. environmental protection agency (epa) tests for chloride contaminants in water is...
Questions in other subjects:

Biology, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00


Mathematics, 09.10.2021 14:00


Mathematics, 09.10.2021 14:00

Business, 09.10.2021 14:00




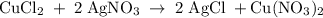
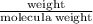
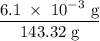
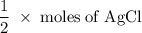
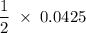 mmoles
mmoles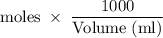
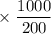 mM
mM




