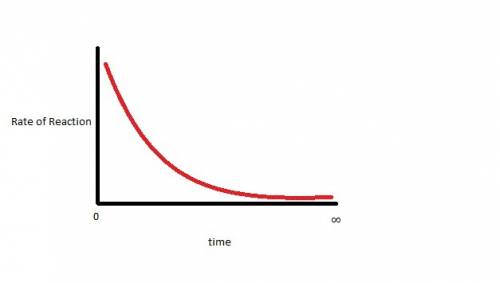
Dinitrogen tetroxide decomposes to produce nitrogen dioxide gas. when dinitrogen tetroxide is sealed in an evacuated glass container, the closed system eventually reaches dynamic equilibrium. which statement describes the graph of the rate of the forward reaction over time? it starts high and gradually decreases until it levels out above zero. it starts high and gradually decreases until it reaches a rate of zero. it starts low and gradually increases until it levels out at a rate above zero. it starts low and gradually increases until it reaches a maximum rate.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
Do you know the correct answer?
Dinitrogen tetroxide decomposes to produce nitrogen dioxide gas. when dinitrogen tetroxide is sealed...
Questions in other subjects:

Mathematics, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40


Social Studies, 20.04.2021 16:40

English, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40

Computers and Technology, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40

English, 20.04.2021 16:40