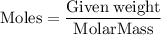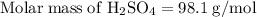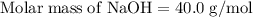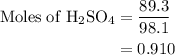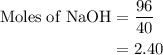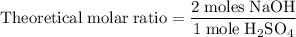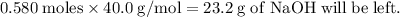Chemistry, 06.10.2019 19:30, lilbrown6369
Aqueous sulfuric acid h2so4 will react with solid sodium hydroxide naoh to produce aqueous sodium sulfate na2so4 and liquid water h2o . suppose 89.3 g of sulfuric acid is mixed with 96. g of sodium hydroxide. calculate the minimum mass of sulfuric acid that could be left over by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, officialgraciela67
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3
Do you know the correct answer?
Aqueous sulfuric acid h2so4 will react with solid sodium hydroxide naoh to produce aqueous sodium su...
Questions in other subjects:





Social Studies, 26.02.2022 01:00


Biology, 26.02.2022 01:00


Social Studies, 26.02.2022 01:00


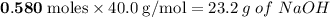 will be left.
will be left.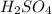 will react with solid sodium hydroxide NaOH to produce aqueous sodium sulfate
will react with solid sodium hydroxide NaOH to produce aqueous sodium sulfate 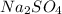 and liquid water
and liquid water 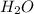 .
.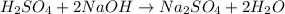
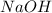 into moles by using the formula,
into moles by using the formula,