
What is the ∆g for the following reaction under standard conditions (t = 298 k) for the formation of nh4no3(s)? 2nh3(g) + 2o2(g) nh4no3(s) + h2o(l) given: nh4no3(s): ∆hf = -365.56 kj ∆sf = 151.08 j/k. nh3(g): ∆hf = -46.11 kj ∆sf = 192.45 j/k. h2o(l): ∆hf = -285.830 kj ∆sf = 69.91 j/k. o2(g): ∆hf = 0.00 kj ∆sf = 205 j/k. 186.6 kj 6.9 kj -10.4 kj -126.3 kj -382 kj

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
Do you know the correct answer?
What is the ∆g for the following reaction under standard conditions (t = 298 k) for the formation of...
Questions in other subjects:





Mathematics, 09.08.2019 00:20





Biology, 09.08.2019 00:20


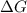 for the reaction is -382 kJ.
for the reaction is -382 kJ.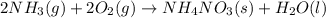
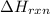 is:
is: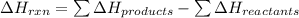
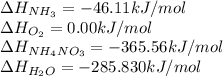
![\Delta H_{rxn}=[1(\Delta H_{NH_4NO_3})+1(\Delta H_{H_2O})]-[2(\Delta H_{NH_3})+2(\Delta H_{O_2})]](/tpl/images/0477/9423/fc8bd.png)
![\Delta H_{rxn}=[1(-365.56)+1(-285.83)]-[2(-46.11)+2(0)]kJ\\\\\Delta H_{rxn}=-559.17kJ=559170J](/tpl/images/0477/9423/fe79f.png)
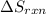 is:
is: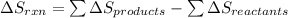
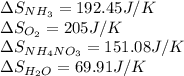
![\Delta S_{rxn}=[1(\Delta S_{NH_4NO_3})+1(\Delta S_{H_2O})]-[2(\Delta S_{NH_3})+2(\Delta S_{O_2})]](/tpl/images/0477/9423/3dbc2.png)
![\Delta S_{rxn}=[1(151.08)+1(69.91)]-[2(192.45)+2(205)]J/K\\\\\Delta S_{rxn}=-573.91J/K](/tpl/images/0477/9423/e9201.png)
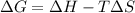
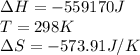
![\Delta G=(-559170J)-[298K\times (-573.91J/K)]\\\\\Delta G=-382kJ](/tpl/images/0477/9423/ce61d.png)




