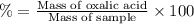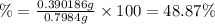Chemistry, 18.10.2019 10:30, anahitrejo1
Oxalic acid is a diprotic acid. calculate the percent of oxalic acid (h2c2o4) in a solid given that a 0.7984-g sample of that solid required 37.98 ml of 0.2283 m naoh for neutralization.

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Oxalic acid is a diprotic acid. calculate the percent of oxalic acid (h2c2o4) in a solid given that...
Questions in other subjects:

Mathematics, 11.10.2019 08:00




History, 11.10.2019 08:00

Social Studies, 11.10.2019 08:00

Mathematics, 11.10.2019 08:00

Geography, 11.10.2019 08:00



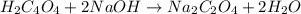
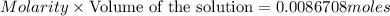
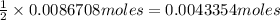 of oxalic acid.
of oxalic acid.