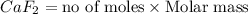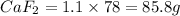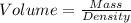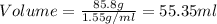Chemistry, 28.01.2020 17:02, jonesromari
How many milliliters of calcium, with a density of 1.55 g/ml, are needed to produce 85.8 grams of calcium fluoride in the single replacement reaction below? show all steps of your calculation as well as the final answer.
unbalanced equation: ca + hf yields caf2 + h2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 01:00, morrisjillian23
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1
Do you know the correct answer?
How many milliliters of calcium, with a density of 1.55 g/ml, are needed to produce 85.8 grams of ca...
Questions in other subjects:

Mathematics, 31.01.2020 17:46

Mathematics, 31.01.2020 17:46


Mathematics, 31.01.2020 17:46


Mathematics, 31.01.2020 17:47

Health, 31.01.2020 17:47

Health, 31.01.2020 17:47

Mathematics, 31.01.2020 17:47


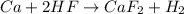
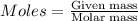
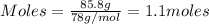
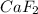 is produced by 1 mole of Ca
is produced by 1 mole of Ca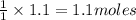 of Ca.
of Ca.