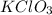Chemistry, 23.09.2019 11:30, nauticatyson9
A13.00 g sample of a compound contains 4.15 g potassium (k), 3.76 g chlorine (cl), and oxygen (o). calculate the empirical formula.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, only1cache
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
Do you know the correct answer?
A13.00 g sample of a compound contains 4.15 g potassium (k), 3.76 g chlorine (cl), and oxygen (o). c...
Questions in other subjects:




Mathematics, 03.03.2021 18:30

History, 03.03.2021 18:30


Mathematics, 03.03.2021 18:30