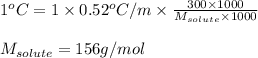Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2


Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
Do you know the correct answer?
What is the approximate molar mass of a molecular solute if 300 g of the solute in 1000 g of water c...
Questions in other subjects:

Mathematics, 23.03.2020 20:33

Mathematics, 23.03.2020 20:33


Health, 23.03.2020 20:33




Business, 23.03.2020 20:33



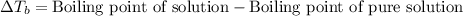
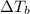 = ? °C
= ? °C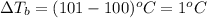
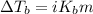
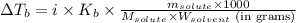
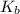 = molal boiling point elevation constant = 0.52°C/m.g
= molal boiling point elevation constant = 0.52°C/m.g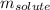 = Given mass of solute = 300 g
= Given mass of solute = 300 g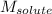 = Molar mass of solute = ?
= Molar mass of solute = ?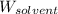 = Mass of solvent (water) = 1000 g
= Mass of solvent (water) = 1000 g