
Chemistry, 26.09.2019 18:30, briannaseaton123
What is the [h3o+] of a solution with a ph of 2.56? 2.56 m, 2.75 x 10-3 m, 363 m, or -0.408 m

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 22:30, needhelpasap8957
Why is the bottom layer of a trophic pyrimid the
Answers: 2

Chemistry, 23.06.2019 06:00, tytianadyson74
What volume of argon gas is equal to 1.60 grams of argon
Answers: 1
Do you know the correct answer?
What is the [h3o+] of a solution with a ph of 2.56? 2.56 m, 2.75 x 10-3 m, 363 m, or -0.408 m...
Questions in other subjects:


Mathematics, 21.03.2021 21:30

Mathematics, 21.03.2021 21:30


Mathematics, 21.03.2021 21:30

World Languages, 21.03.2021 21:30

Mathematics, 21.03.2021 21:30


Mathematics, 21.03.2021 21:30


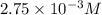 .
.![pH=-\log[H_3O^+]](/tpl/images/0265/4012/a23b5.png)
![2.56=-\log[H_3O^+]](/tpl/images/0265/4012/79520.png)
![[H_3O^+]=0.002754 M=2.75\times 10^{-3} M](/tpl/images/0265/4012/b3edb.png)




