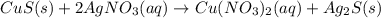Chemistry, 15.11.2019 20:31, Tringirl233
E. solid copper sulfide and silver nitrate react to form copper (ii) nitrate and solid silver sulfide. write a balanced chemical equation that describes the reaction. identify the oxidation number of each element in the reaction. (you do not need to include the total contribution of charge.) is this reaction a redox reaction or a non-redox reaction? explain your answer.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 07:30, sweetLips230
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1

Do you know the correct answer?
E. solid copper sulfide and silver nitrate react to form copper (ii) nitrate and solid silver sulfid...
Questions in other subjects:



Biology, 02.03.2021 03:00



Biology, 02.03.2021 03:00


Mathematics, 02.03.2021 03:00

Mathematics, 02.03.2021 03:00