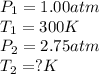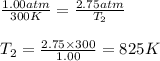Aflask of fixed volume contains 1.00 mole of gaseous carbon dioxide and 88.0 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.00 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.75 atm. what is the final temperature in kelvins? assume the solid carbon dioxide takes up negligible volume.

Answers: 1
Similar questions

Chemistry, 22.06.2019 11:00, daniel1480
Answers: 2

Chemistry, 27.06.2019 01:30, edjiejwi
Answers: 1

Chemistry, 26.08.2019 19:30, ericgalo808
Answers: 1

Chemistry, 05.11.2019 02:31, knela
Answers: 1
Do you know the correct answer?
Aflask of fixed volume contains 1.00 mole of gaseous carbon dioxide and 88.0 g of solid carbon dioxi...
Questions in other subjects:

English, 09.10.2019 09:00

Mathematics, 09.10.2019 09:00


English, 09.10.2019 09:00


Mathematics, 09.10.2019 09:00

Mathematics, 09.10.2019 09:00

English, 09.10.2019 09:00


Mathematics, 09.10.2019 09:00


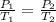 (at constant volume)
(at constant volume)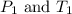 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.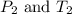 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.