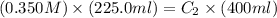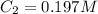Chemistry, 24.12.2019 19:31, odymonster9
Initially a beaker contains 225.0 ml of a 0.350 m mgso4 solution. then 175.0 ml of water are added to the beaker. find the concentration of the final solution. what is the volume?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
Do you know the correct answer?
Initially a beaker contains 225.0 ml of a 0.350 m mgso4 solution. then 175.0 ml of water are added t...
Questions in other subjects:


Computers and Technology, 27.04.2021 01:00


English, 27.04.2021 01:00

Physics, 27.04.2021 01:00





Mathematics, 27.04.2021 01:00


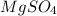 + Volume of water added
+ Volume of water added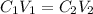
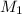 = concentration of
= concentration of 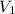 = volume of
= volume of 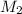 = concentration of final solution = ?
= concentration of final solution = ?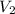 = volume of final solution = 400 ml
= volume of final solution = 400 ml