Why is water considered a polar molecule?
the molecule has no overall charge.
the...


Answers: 1
Similar questions

Biology, 05.07.2019 00:40, prince8522
Answers: 1

Physics, 23.07.2019 09:00, mileto5824
Answers: 1

Biology, 19.09.2019 18:30, kee387
Answers: 1

Physics, 11.10.2019 04:00, karatsgrande3772
Answers: 2
Do you know the correct answer?
Questions in other subjects:



English, 02.09.2019 14:50

History, 02.09.2019 14:50





History, 02.09.2019 14:50


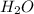 . As oxygen is more electronegative than hydrogen atom hence, it will attract the electrons more towards itself.
. As oxygen is more electronegative than hydrogen atom hence, it will attract the electrons more towards itself. 




