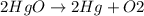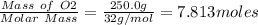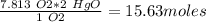Chemistry, 24.09.2019 11:30, vickygloom
Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equation is shown below. 2hgo mc020-1.jpg 2hg + o2 the molar mass of hgo is 216.59 g/mol. the molar mass of o2 is 32.00 g/mol. how many moles of hgo are needed to produce 250.0 g of o2?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Do you know the correct answer?
Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equat...
Questions in other subjects:

Mathematics, 29.10.2020 04:00


English, 29.10.2020 04:00

Mathematics, 29.10.2020 04:00


Arts, 29.10.2020 04:00

English, 29.10.2020 04:00

Spanish, 29.10.2020 04:00

Mathematics, 29.10.2020 04:00

Computers and Technology, 29.10.2020 04:00