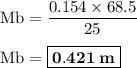The concentration of the HCl solution : 0.421 m
Further explanation
Titration is a procedure for determining the concentration of a solution by reacting with another solution which is known to be concentrated (usually a standard solution). Determination of the endpoint/equivalence point of the reaction can use indicators according to the appropriate pH range
Titrations can be distinguished including acid-base titration, depositional titration, and redox titration. An acid-base titration is the principle of neutralization of acids and bases is used.
An acid-base titration there will be a change in the pH of the solution.
From this pH change a Titration Curve can be made which is a function of acid / base volume and pH of the solution
Acid-base titration formula
Ma. Va. na = Mb. Vb. nb
Ma, Mb = acid base concentration
Va, Vb = acid base volume
na, nb = acid base valence
Neutralization Reaction :
NaOH + HCl ⇒ NaCl + H₂O
68.5 ml of 0.154 m NaOH
25.0 ml of HCl
Acid-base titration formula
Ma. Va. na = Mb. Vb. nb
a = NaOH, b = HCl(both have valence 1)
0.154 m . 68.5.1 = Mb . 25 . 1
Learn more
the best tool to find the volume of that she needs to neutralize the acid
link
Determine the endpoint of the titration.
link
acid-base
link
Identify the acids and the bases
link
endpoint titration
link