
Consider the following element combinations. classify the bonds formed between each pair as ionic, polar covalent, or nonpolar covalent qualitatively based solely on each element's position on the periodic table? do not conduct calculations. 1. rb and i 2. br and br 3. s and i 4. n and n 5. o and f 6. cs and cl 7. ca and o 8. p and i

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3

Chemistry, 23.06.2019 05:00, lraesingleton
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
Do you know the correct answer?
Consider the following element combinations. classify the bonds formed between each pair as ionic, p...
Questions in other subjects:

Mathematics, 11.02.2022 01:00

English, 11.02.2022 01:00

English, 11.02.2022 01:00








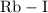 is an
is an 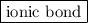 .
.
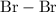 is a
is a 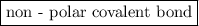 .
.
 is a
is a 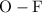 is a
is a 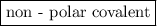 .
.
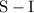 is a
is a  is an
is an  is a
is a 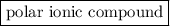 .
.
 is a
is a 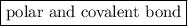 .
.
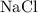 is an ionic compound.
is an ionic compound.
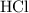 . Here the bond is polar and covalent. Another example of covalent molecule is methane where the difference in the C-H electronegativities is very small so it regarded as a pure covalent molecule.
. Here the bond is polar and covalent. Another example of covalent molecule is methane where the difference in the C-H electronegativities is very small so it regarded as a pure covalent molecule.
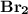 is a non-polar covalent bond. Similarly
is a non-polar covalent bond. Similarly 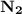 is a non-polar covalent molecule.
is a non-polar covalent molecule.
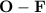 bond are non-polar covalent bonds.
bond are non-polar covalent bonds.
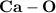 is a polar covalent bond.
is a polar covalent bond.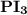 is regarded as polar and covalent bonds.
is regarded as polar and covalent bonds.




