
Chemistry, 20.09.2019 14:00, masonprice
A0.00100 mol sample of ca(oh)2 requires 25.00 ml of aqueous hcl for neutralization according to the reaction below. what is the concentration of the hcl? ca(oh)2(s) + 2hcl(aq) --> cacl2(aq) + h2o(l)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Do you know the correct answer?
A0.00100 mol sample of ca(oh)2 requires 25.00 ml of aqueous hcl for neutralization according to the...
Questions in other subjects:


Mathematics, 14.04.2020 19:40


Biology, 14.04.2020 19:40







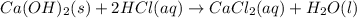
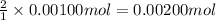
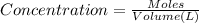
![[HCl]=\frac{0.00200 mol}{0.025 L}=0.08 mol/L](/tpl/images/0246/4244/941e7.png)




