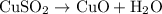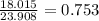Chemistry, 16.09.2019 18:40, juicemankinnie95
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cuo (mw = 79.545 g/mol) produced. given that 3.327 g of cuo was produced during the reaction, how many grams of water were released as water vapor? (number of moles = mass (g) / molar mass (g/ choose the closest answer.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Do you know the correct answer?
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cu...
Questions in other subjects:


English, 12.10.2019 10:10


Mathematics, 12.10.2019 10:10