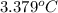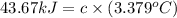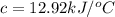Chemistry, 26.09.2019 22:40, kraigstlistt
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol. when 1.411 g of compound a (molar mass = 115.27 g/mol) was burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.379 °
c. using this data, what is the heat capacity (calorimeter constant) of the calorimeter?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2


Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
Do you know the correct answer?
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol....
Questions in other subjects:


History, 22.05.2020 11:00

Mathematics, 22.05.2020 11:00



Mathematics, 22.05.2020 11:00

History, 22.05.2020 11:00



Mathematics, 22.05.2020 11:00


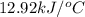
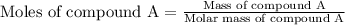
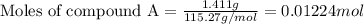
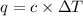
 = change in temperature =
= change in temperature =