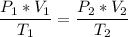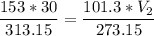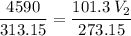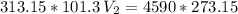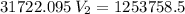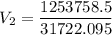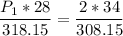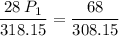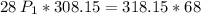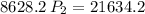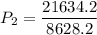Chemistry, 05.10.2019 09:01, zhellyyyyy
The volume of a gas-filled balloon is 30.0 l at 40 °c and 153 kpa pressure. what volume will the balloon have at standard temperature and pressure (273.15 k and 101.3 kpa)?
a. 17.3 l
b. 23.7 l
c. 39.5 l
d. 51.9 l
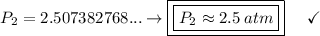
a gas that has a volume of 28 liters, a temperature of 45 °c, and an unknown pressure, has its volume increased to 34 liters and its temperature decreased to 35 °c. if i measure the pressure after the change to be 2.0 atm, what was the original pressure of the gas?
a. 1.5 atm
b. 1.7 atm
c. 2.8 atm
d. 2.5 atm

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 01:30, rubyr9975
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
Do you know the correct answer?
The volume of a gas-filled balloon is 30.0 l at 40 °c and 153 kpa pressure. what volume will the bal...
Questions in other subjects:


Social Studies, 21.10.2020 01:01

Health, 21.10.2020 01:01


Geography, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01