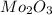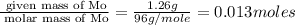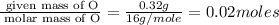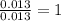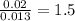Acompound that is composed of molybdenum (mo) and oxygen (o) was produced in a lab by heating molybdenum over a bunsen burner. the following data was collected:
mass of crucible: 38.26 g
mass of crucible and molybdenum: 39.52 g
mass of crucible and molybdenum oxide: 39.84 g
solve for the empirical formula of the compound, showing your calculations.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
Do you know the correct answer?
Acompound that is composed of molybdenum (mo) and oxygen (o) was produced in a lab by heating molybd...
Questions in other subjects:


English, 17.10.2020 07:01


Mathematics, 17.10.2020 07:01



Mathematics, 17.10.2020 07:01

Advanced Placement (AP), 17.10.2020 07:01