
Arigid, 2.50 l bottle contains 0.458 mol he. the pressure of the gas inside the bottle is 1.83 atm. if 0.713 mol ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of the gas mixture? the final temperature of the gas is k.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
Do you know the correct answer?
Arigid, 2.50 l bottle contains 0.458 mol he. the pressure of the gas inside the bottle is 1.83 atm....
Questions in other subjects:

Mathematics, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

Chemistry, 11.09.2020 04:01

Biology, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

Arts, 11.09.2020 04:01

English, 11.09.2020 04:01

Social Studies, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01


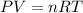
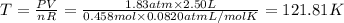
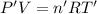
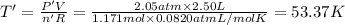
 2.50 = 0.458
2.50 = 0.458 



