
Chemistry, 02.10.2019 18:30, maxraph108
Asolution is prepared by dissolving 23.7 g of cacl2 in 375 g of water. the density of the resulting solution is 1.05 g/ml. the concentration of cacl2 in this solution is molar.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2


Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
Do you know the correct answer?
Asolution is prepared by dissolving 23.7 g of cacl2 in 375 g of water. the density of the resulting...
Questions in other subjects:




Mathematics, 28.06.2021 04:10





Mathematics, 28.06.2021 04:20


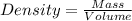
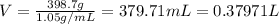
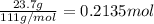
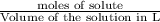
![[CaCl_2]=\frac{0.2135 mol}{0.37971 L}=0.5623 molar](/tpl/images/0283/7402/17e4b.png)




