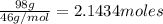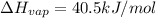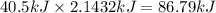Chemistry, 02.10.2019 10:50, moniquejg1800
How much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhvap is 40.5 kj/mol? how much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhvap is 40.5 kj/mol? 52.8 kj 11.5 kj 86.7 kj 39.9 kj 18.9 kj

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, btcastongia
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 17:30, destineysarah
98 points you will be galileo perform the experiment to determine if objects with different mass fall at the same, or different, rates in the air and in a vacuum. before you conduct your experiment, you need to form a hypothesis. a hypothesis is a prediction of what you think will happen in the experiment. the hypothesis is a statement that describes “if” a certain set of circumstances are present “then” there will be a specific result that will occur. record your hypothesis here: record the results from step one of the experiment (dropping the objects in the air): first trial: second trial: third trial: record the results from step two of the experiment (dropping the objects in a vacuum): first trial: second trial: third trial: did the experiment support your hypothesis? using the data from your experiment, describe why you believe your hypothesis was either proven or disproven. what forces were acting on the objects dropped in the air? what force was acting on the objects dropped in the vacuum? part two: comparing forces choose two forces and compare and contrast these forces. you must provide two ways that they are alike and two ways that they are different. you may make a list, write in paragraph form, or make a chart. choose two forces and compare and contrast these forces. these must be different forces than used in the prior question. provide two ways that they are similar and two ways that they are different. you may make a list, write it out, or make a chart.
Answers: 3

Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
Do you know the correct answer?
How much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhva...
Questions in other subjects:


History, 18.01.2021 14:00

Chemistry, 18.01.2021 14:00

Physics, 18.01.2021 14:00

English, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00


English, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00